The speakers selected for the panel:
- Bassil Akra, Ph.D., CEO, Akra Team GmbH
- Joao Martins, Pharm.D., Associate Director Regulatory Strategy, Abbott
- Matthias Fink, M.D., Clinical Focus Team Manager, TÜV SÜD America
- Robert Dostert, Sales Manager, DNV
- Lana Feng, Ph.D., CEO, and Co-founder of Huma.AI
During the webinar, moderator Bassil Akra, Ph.D., discussed the lack of resources available to perform the tasks for the Post-Market Surveillance (PMS) requirements of EU MDR and IVDR. With talent available only at a premium, MedTech companies are struggling to meet the demands of necessary processes in a way that is efficient and cost-effective.
Device Class Determines PMS Requirements
Dr. Joao Martins, Pharm.D., Associate Director Regulatory Strategy at Abbott, covered the differences in PMS requirements based on the class of a given device. That class determines the extensiveness of PMS requirements, as seen in Figure 1.
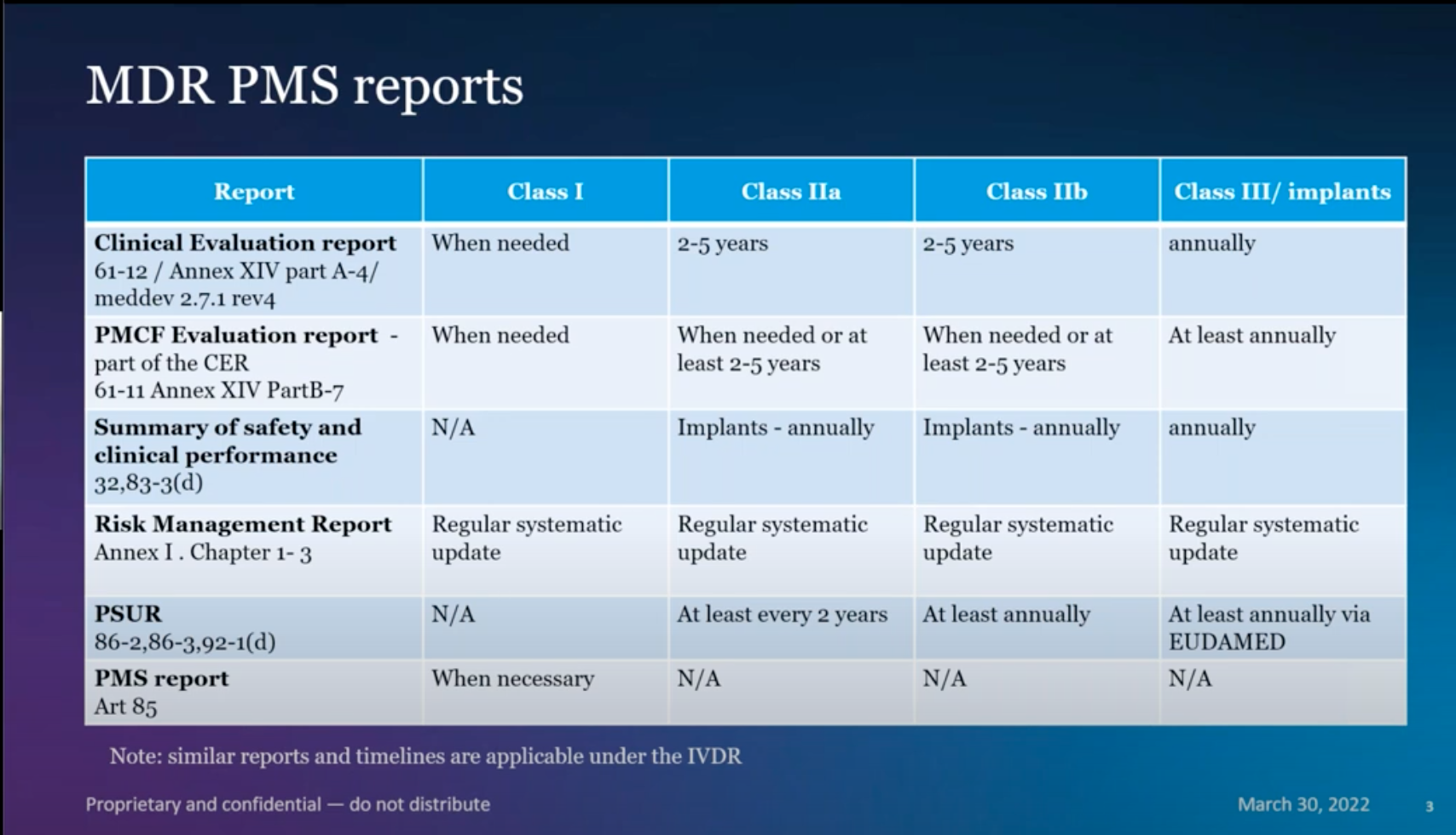
Figure 1 - MDR PMS Reports by Class
Later in the Webinar, Dr. Martins described the limited resources available to perform the work necessary for PMS - how alternative approaches that involve Machine Learning (ML) technology. He stated:
“Certainly the use of options that can optimize processes and make them be more efficient is one of the ways to go. In many cases, throwing resources at the problem does not necessarily solve the problem. You've got to better structure your process. You've got to make sure that you have an efficient process. If you do not, also look at the tools that you can use to make it more efficient. Certainly machine learning, or any activity or tool that can optimize a process, is a way to go.”
Understanding the Challenges of PMCF Plans
In his presentation and during the concluding discussion, Dr. Fink outlined the comprehensive, interdependent nature of Post-Market Clinical Follow-up (PMCF) plans at length, based on multiple clarification questions about that topic:
“The MDR has a little bit of a broader definition of post-market clinical follow-up. As we see in Annex 14, 6.2, for the requirements of the PMCF plan, the MDR differentiates between general methods like clinical research, post-market feedback, complaints, and use of feedback, which under the MDD were more considered general PMS activities. They differentiate [requirements] into specific PMCF activities, which could be, for example, PMCF studies, registries, or specific surveys. Also, it's required, and this is very important, [for there to be] a rationale for the appropriateness of the method.”
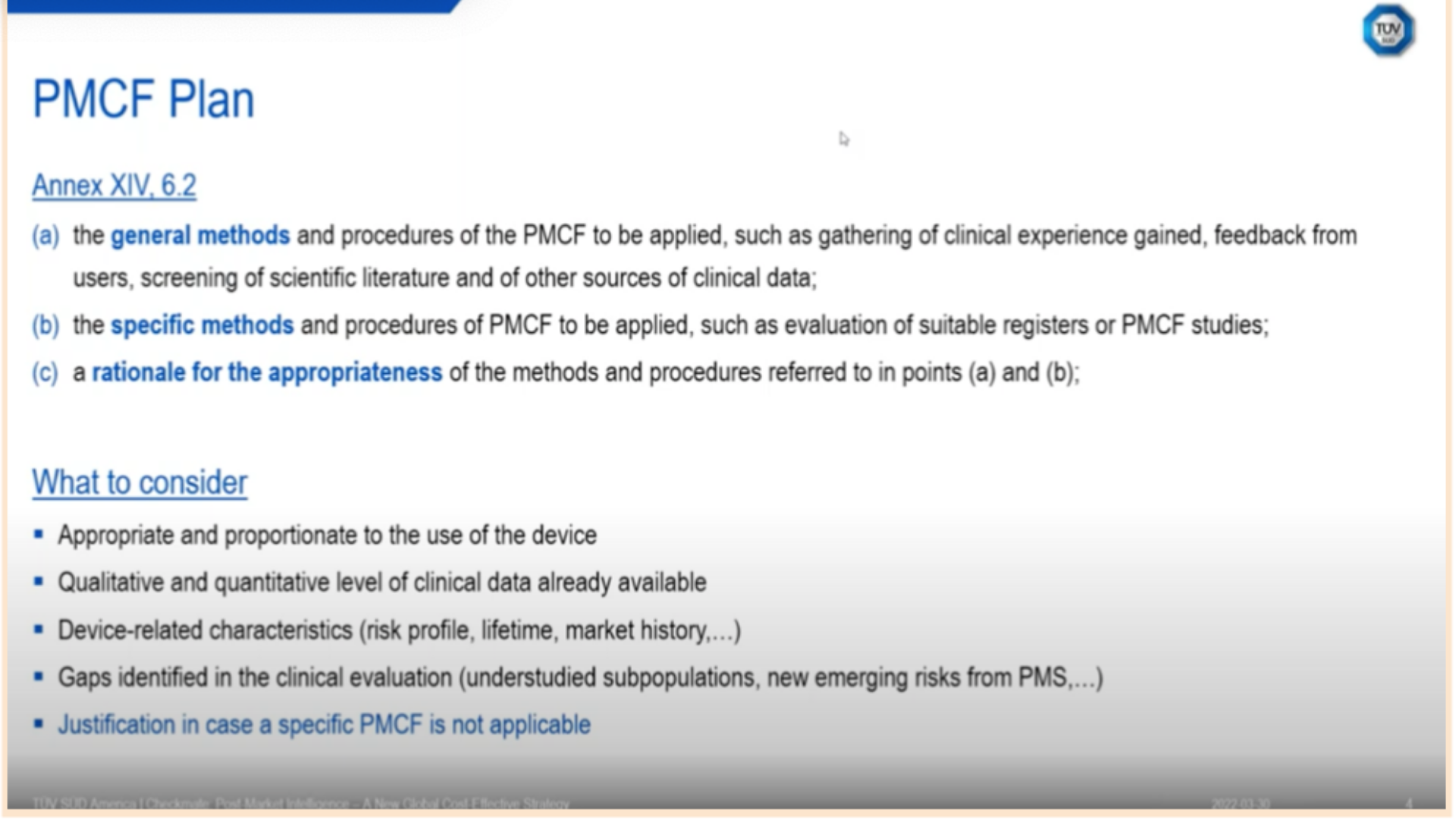 Figure 2 - PMCF Plan
Figure 2 - PMCF Plan
Picking up where Dr. Martins left off, Dr. Feng further explored augmenting human experts in PMS with Machine Learning, in part because of a lack of resources available for PMS compliance.
“We can use technology to allow you to do more with less…It's really easy for machines to mimic some of the manual work that humans do. [They are] able to automate it and increase it.”
The poll findings were in line with the discussion of limited resources and augmentation. The number one response to the question, “How do you plan to solve PMS challenges?” was to allocate more resources (49%).
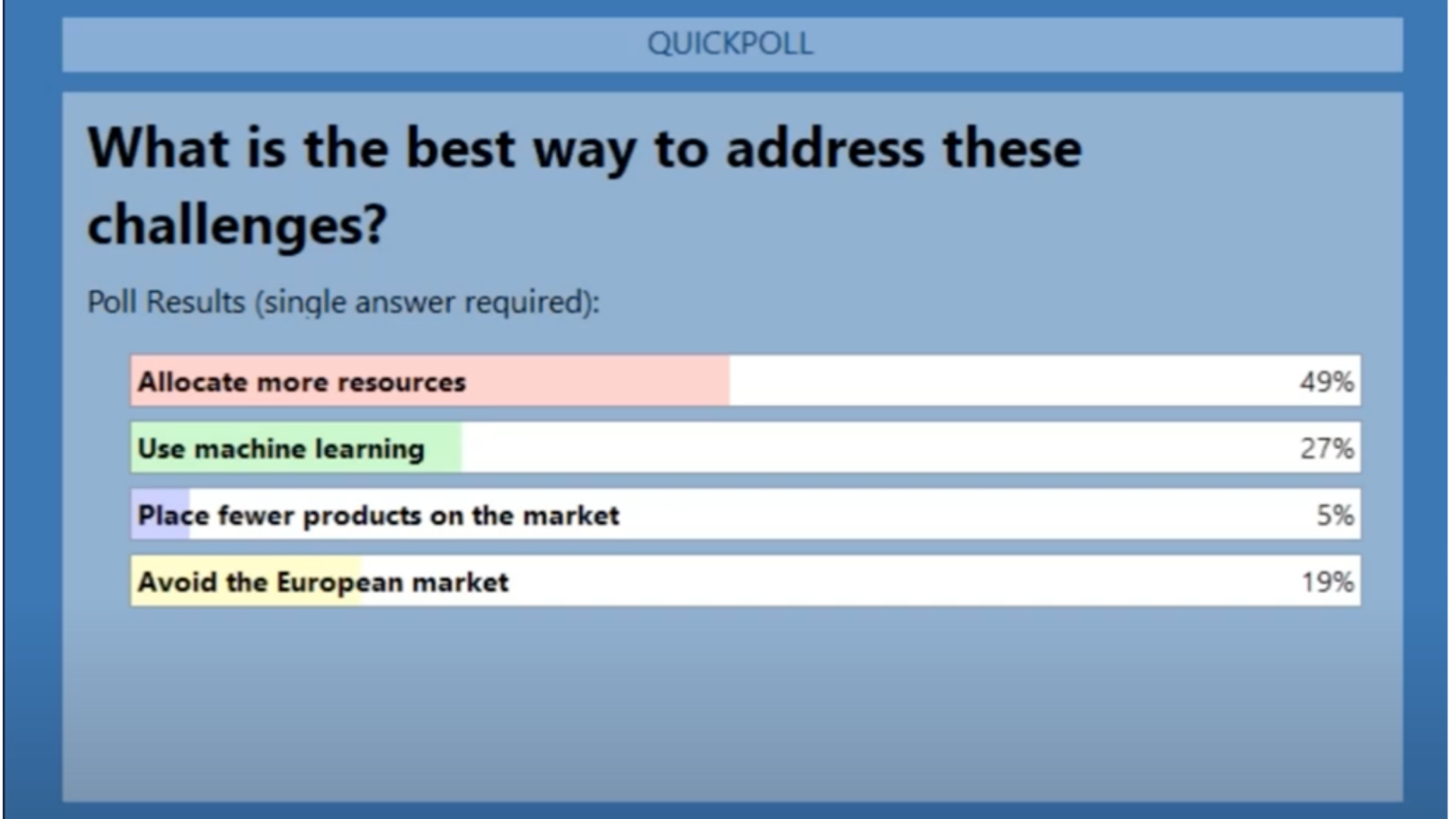
Figure 3 - Webinar Poll Results
Machine Learning Is the Answer
Following the preferred path of allocating more resources is cost-prohibitive and not always possible. The shortage of talent available, even at a premium, to do the work necessary for PMS compliance, does not look to end any time soon.
The second highest answer offers more hope. Those who expressed an interest in machine learning (27%) have an opportunity to meet the challenges of MDR and IVDR discussed by Dr. Akra, Dr. Martins, and Dr. Feng by augmenting the work of existing teams with the services of a technology partner. Overall, the panel’s discussion drove home the value of collaboration between humans and Machine Learning, finding it essential to navigating the complex transition to MDR and IVDR compliance.
* * *
To learn more about streamlining your IVDR compliance processes, request a discovery call and demo below.
.png?width=300&height=120&name=Huma-AI-Logo-(Horizontal).png)



.png?width=200&name=Huma-AI-Logo-(Horizontal-Reverse).png)